Atomic Timeline
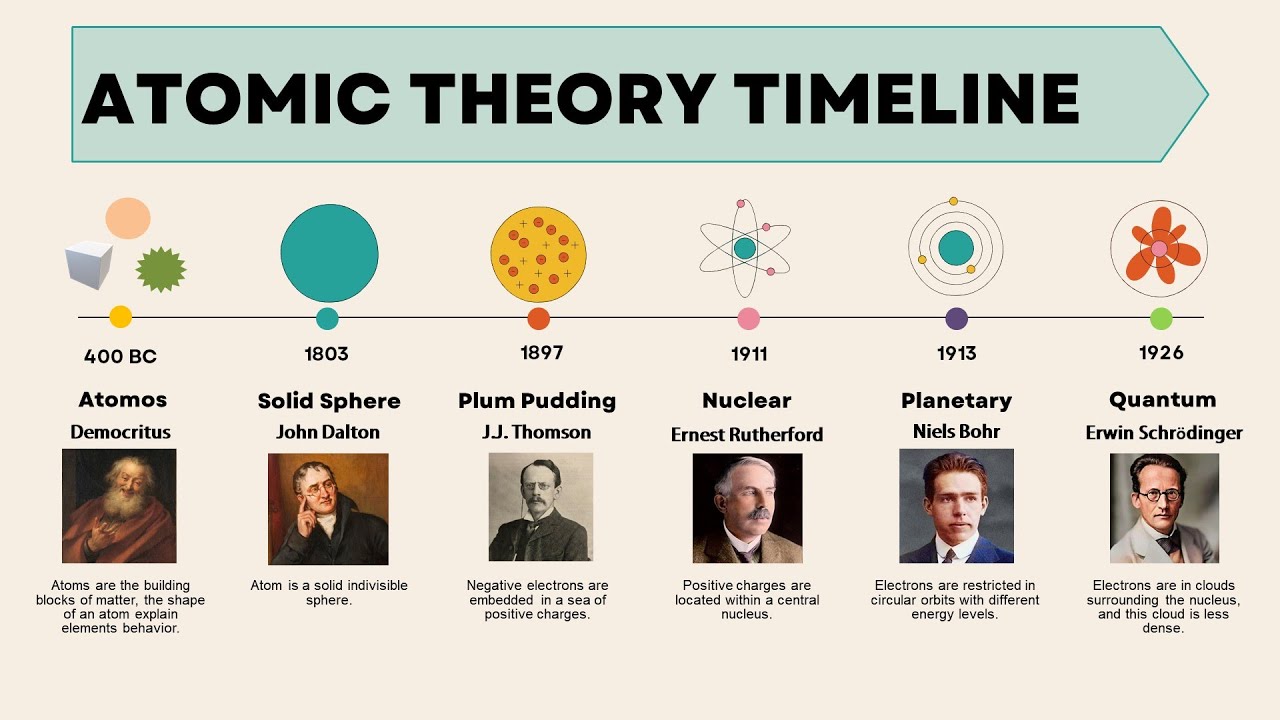
From ancient philosophical ponderings to groundbreaking scientific discoveries, the journey of humanity’s understanding of the atomic realm has been a fascinating odyssey. The atomic timeline represents a chronicle of milestones, each marking significant advancements in our comprehension of the fundamental building blocks of matter. Embark with us on this voyage through time as we unravel the story of atoms and the scientific endeavors that have shaped our modern understanding.
Ancient Philosophies and Early Theories:
The journey of atomic exploration dates back to antiquity, where philosophers in various civilizations speculated on the nature of matter. Among them, the ancient Greeks made significant contributions, with figures like Democritus proposing the concept of indivisible particles called “atomos,” from which the term “atom” originates. However, these early theories lacked empirical evidence and remained speculative for centuries.
Alchemy and Early Chemistry:
The medieval era witnessed the rise of alchemy, a precursor to modern chemistry, which sought to transform base metals into noble ones and discover the philosopher’s stone. While alchemists delved into experimental practices, their understanding of matter remained largely mystical and speculative. It wasn’t until the Scientific Revolution of the 17th century that a more systematic approach to studying atoms emerged.
The Birth of Modern Atomic Theory: In the early 19th century, John Dalton formulated the first modern atomic theory, postulating that all matter is composed of indivisible atoms, each with its own unique properties. Dalton’s atomic theory provided a framework for understanding chemical reactions and laid the foundation for subsequent scientific inquiries into the nature of atoms.
Discovery of Subatomic Particles:
The late 19th and early 20th centuries witnessed remarkable discoveries that shattered the notion of atoms as indivisible entities. J.J. Thomson’s experiments with cathode rays led to the identification of the electron, a negatively charged subatomic particle. This discovery challenged Dalton’s original conception of atoms and opened the door to further exploration of atomic structure.
Ernest Rutherford’s famous gold foil experiment provided compelling evidence for the existence of a dense nucleus within atoms, surrounded by a cloud of electrons. Rutherford’s model of the atom depicted a small, positively charged nucleus orbited by negatively charged electrons, resembling the structure of a miniature solar system.
The Quantum Revolution:
The early 20th century also saw the rise of quantum mechanics, a revolutionary theory that transformed our understanding of atomic and subatomic phenomena. Pioneered by luminaries such as Max Planck, Albert Einstein, Niels Bohr, and Werner Heisenberg, quantum mechanics introduced probabilistic descriptions of particle behavior and challenged classical notions of determinism.
Bohr’s model of the hydrogen atom, incorporating quantized electron orbits and the emission of discrete energy packets (quanta) upon electron transitions, provided a quantum-mechanical explanation for the spectral lines observed in atomic spectra. This model laid the groundwork for future advancements in atomic physics and quantum theory.
The Standard Model of Particle Physics:
In the latter half of the 20th century, the development of particle accelerators and advanced experimental techniques facilitated the discovery of a plethora of subatomic particles. The Standard Model of particle physics emerged as a comprehensive framework that described the fundamental constituents of matter and their interactions via three fundamental forces: electromagnetism, the weak nuclear force, and the strong nuclear force.
Key milestones in this era include the discovery of the neutron by James Chadwick, the elucidation of the quark model by Murray Gell-Mann and George Zweig, and the confirmation of the Higgs boson’s existence at the Large Hadron Collider in 2012. These discoveries have deepened our understanding of the subatomic realm and reinforced the predictive power of the Standard Model.
Technological Applications and Implications:
The journey through the atomic timeline isn’t merely a historical account but also a testament to the transformative impact of atomic science on society. From nuclear energy and medical imaging to semiconductor technology and materials science, atomic discoveries have catalyzed innovations that have revolutionized countless industries and improved human welfare.
However, alongside the benefits, atomic science also raises ethical and existential concerns, particularly regarding nuclear weapons proliferation, radioactive waste disposal, and the potential consequences of atomic accidents. As we continue to harness the power of atoms for societal advancement, it’s imperative to confront these challenges with responsible stewardship and ethical foresight.
Conclusion:
The atomic timeline represents a tapestry of human ingenuity and curiosity, woven through centuries of scientific inquiry and discovery. From ancient speculations to modern particle accelerators, each milestone illuminates another facet of the intricate dance of atoms and the underlying laws governing their behavior. As we stand on the precipice of a new era of atomic exploration, let us carry forward the spirit of curiosity and collaboration that has propelled us thus far, ever mindful of the profound implications of our atomic discoveries on the future of humanity and the cosmos.





